Research
Systems Immunology At The Bedside

Mass Cytometry (CyTOF) in Clinical Studies
High-parameter, single-cell technologies are revolutionizing the fields of human immunology and precision medicine. The lab employs a multidisciplinary approach involving deep immune profiling of patient samples and customized computational methods to link high-dimensional immune datasets to precisely defined clinical outcomes. The Gaudillière Laboratory is integrated within a larger collaborative consortium with expertise in bioinformatics (Dr. Nima Aghaeepour’s laboratory), clinical trials (Dr. Martin Angst’s laboratory), and Immunology (Dr. Garry Nolan’s laboratory).
Data Integration Across Omics Platforms
The immune system is involved in a symbiotic interplay with various biological and environmental factors. To understand the immune system in a clinical context these relationships must be characterized accurately. We develop integrative statistical models that bring together various datasets (including from the transcriptome, metabolome, and microbiome) of patients to both more accurately diagnose diseases and to understand their underlying biological mechanisms. This effort is primarily led by the laboratory of Dr. Nima Aghaeepour.
Ongoing Clinical Studies
Trauma and Surgery
How did our ancestors survive after being attacked by a saber-toothed cat? The immune system has evolved sophisticated cellular programs that enable us to heal and recover from major trauma. We study patients undergoing surgery, which is a useful model of traumatic injury because we can interrogate their immune system “before” the injury ever happens. Using high-dimensional mass cytometry, we have recently shown that the signaling behavior of a network of innate immune cells measured before surgery predicted surgical recovery. We are now in the process of prospectively connecting these predictions to measurements from wearable devices to provide patients with real-time feedback. Ongoing work in humans and animal models focuses on pre-operative interventions (“pre-hab”) that alter a patient’s immune state to improve recovery after surgery.

Pregnancy
Human pregnancies last precisely nine months. To achieve this precision in timing, mothers rely on a finely-tuned immune balance (or immune clock), which controls an immunological switch from tolerance to rejection of the fetus (a hemi-allogeneic foreign body). In collaboration with the March of Dimes and the Bill and Melinda Gates’ foundations, the lab studies the adaptation of feto-maternal immunity to normal and abnormal pregnancies (e.g. associated with preterm birth and preeclampsia). Using an multi-disciplinary approach that integrates deep immune profiling of pregnancy with changes in cell-free RNA expression (collaboration with Dr. Steve Quake’s lab), proteomics (Dr. Martin Angst's lab), metabolomics (Dr. Mike Snyder's lab) and the maternal microbiota (Dr. David Relman’s lab), our goal is to identify peripheral blood signatures that predict pregnancy complications.
COVID Pandemic
Why do some who become exposed to SARS-CoV-2 become critically ill, while others have mild to moderate symptoms? Some of the greatest medical challenges of COVID-19 disease are owed to the highly unpredictable progression from mild to severe symptoms. Data from the SARS epidemic and emerging data from the SARS-CoV-2 pandemic suggest that in conjunction with profound adaptive immune system suppression, exacerbated innate immune responses to SARS-CoV-2 are key factors in the pathobiology of severe disease progression. In collaboration with Dr. Kari Nadeau’s laboratory (Stanford University) and the COVID-19 unit of the Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán in Mexico City (Dr. Sergio Valdez, and Dr. Jose Crispin), the Gaudilliere lab will investigate the host response to SARS-CoV-2 infection. Using high-dimensional mass cytometry and proteomics, circulating inflammatory factors combined with functional analysis of innate and adaptive immune responses will be assessed to identify predictors of disease severity and clinical outcomes.
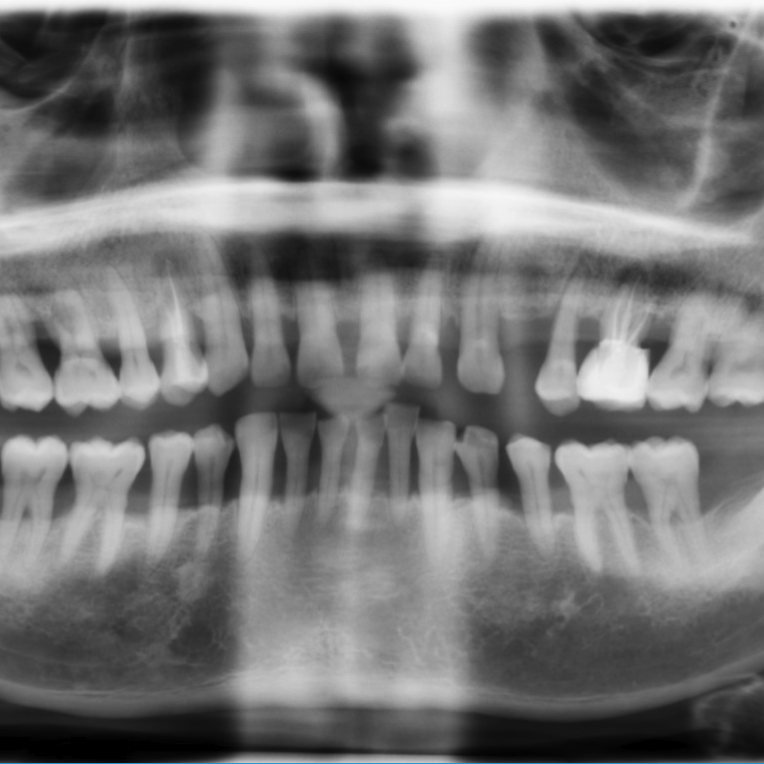
Periodontal Disease
Periodontitis, one of the most prevalent chronic disease, is a microbe-mediated immuno-inflammatory pathology leading to the degeneration of tissues supporting the teeth. It has been presented as a comorbidity with several systemic issues, including cardiovascular disease, diabetes, certain malignancies, inflammatory bowel disease, and adverse pregnancy outcomes. The periodontium presents a large, inflamed surface area that is rich in dysbiotic microbes leading to chronic low-grade systemic inflammation. Using high-dimensional mass cytometry, our goal is to identify a systemic immune signature of periodontitis in order to investigate further intervention strategies to control the systemic impact of this inflammatory state.
Stroke
Stroke is the number one cause of long-term disability in the world. Acute stroke elicits a profound systemic inflammatory response, not unlike traumatic injury. We are interested in early immunological mechanisms, mobilized hours to days after the ischemic event, that predict patients’ long-term neurocognitive recovery.

Neonatology
Immune dysregulation is at the heart of many of the diseases of the newborn—particularly the premature newborn. These babies suffer from an increased vulnerability to infection due to immune compromise while also being susceptible to the runaway inflammation responsible for diseases like bronchopulmonary dysplasia, retinopathy of prematurity, and necrotizing enterocolitis, among others. Together these diseases constitute the majority of morbidities suffered by premature infants. A comprehensive and holistic view of the neonatal immune system’s phenotypic and functional attributes in this population is paramount to being able to predict, prevent, and treat these morbidities. The Gaudilliere lab is using advanced technologies such as mass cytometry to evaluate the dynamics of the newborn immune system from the time of birth onward.

Microgravity and Space Medicine
Space travel is around the corner! For thousands of years, our immune system has evolved here on earth, under 1g gravity. What happens when gravity disappears? Accumulating data from long-duration space flights suggests astronauts’ immune systems are suppressed in space. This has both short- and long-term consequences to their health, with increased predisposition to opportunistic infection and perhaps cardiovascular disease. Our lab has launched a collaboration with Millie Hughes-Fulford (former STS-40 Spacelab Life Sciences astronaut [LINK], now a Professor at UCSF) to understand the adaptation of the human immune system to long-term zero-gravity exposure. Don’t we all dream about seeing someone live on Mars one day?


